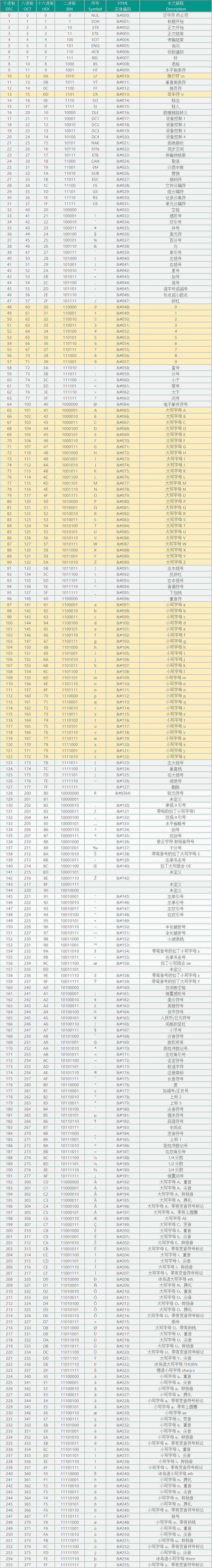
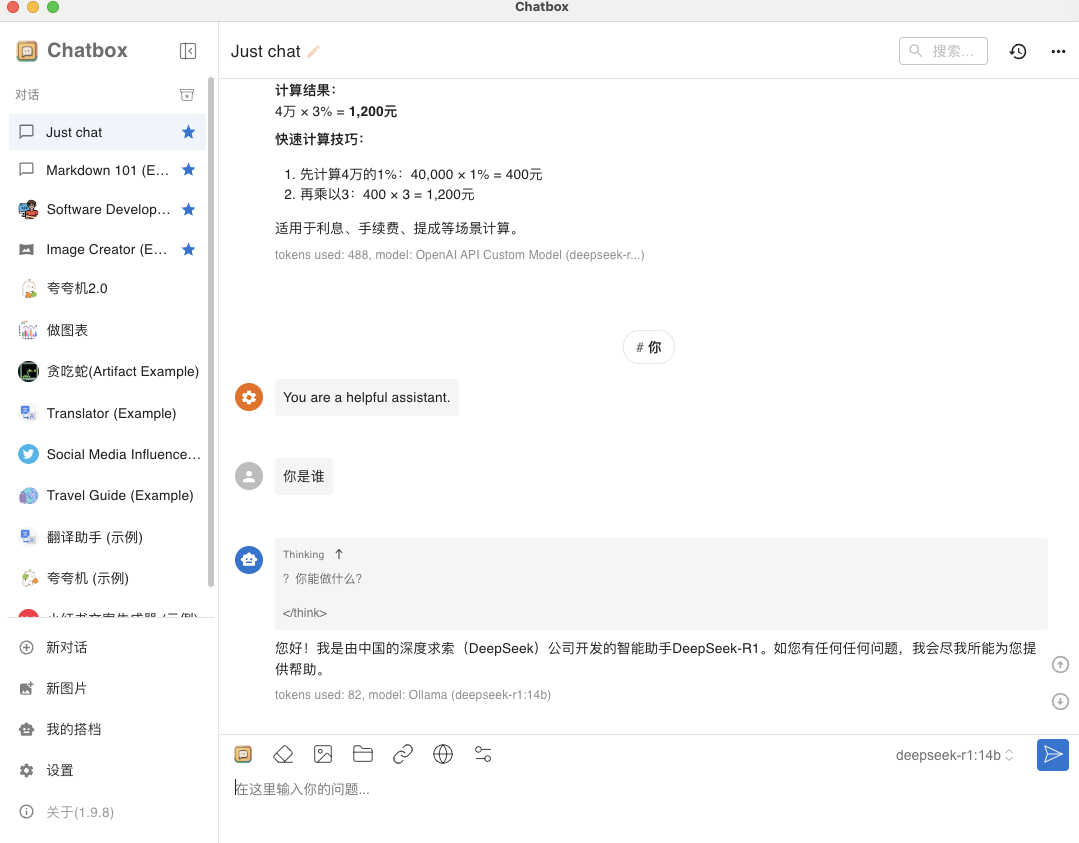
| 物质名称 | 化学式 | 浓度积常数 $ K_{sp} $ |
|---|---|---|
| 氯化银 | \(\text{AgCl}\) | \(1.8 \times 10^{-10}\) |
| 溴化银 | \(\text{AgBr}\) | \(5.0 \times 10^{-13}\) |
| 碘化银 | \(\text{AgI}\) | \(8.5 \times 10^{-17}\) |
| 铬酸银 | \(\text{Ag}_2\text{CrO}_4\) | \(1.1 \times 10^{-12}\) |
| 碳酸钡 | \(\text{BaCO}_3\) | \(8.1 \times 10^{-9}\) |
| 铬酸钡 | \(\text{BaCrO}_4\) | \(2.0 \times 10^{-10}\) |
| 硫酸钡 | \(\text{BaSO}_4\) | \(1.1 \times 10^{-10}\) |
| 碳酸钙 | \(\text{CaCO}_3\) | \(3.4 \times 10^{-9}\) |
| 硫酸钙 | \(\text{CaSO}_4\) | \(4.9 \times 10^{-5}\) |
| 硫化铜 | \(\text{CuS}\) | \(8.0 \times 10^{-37}\) |
| 硫化亚铜 | \(\text{Cu}_2\text{S}\) | \(2.5 \times 10^{-48}\) |
| 氯化亚铜 | \(\text{CuCl}\) | \(1.2 \times 10^{-6}\) |
| 溴化亚铜 | \(\text{CuBr}\) | \(6.3 \times 10^{-9}\) |
| 碘化亚铜 | \(\text{CuI}\) | \(1.1 \times 10^{-12}\) |
| 氢氧化亚铁 | \(\text{Fe(OH)}_2\) | \(4.9 \times 10^{-17}\) |
| 氢氧化铁 | \(\text{Fe(OH)}_3\) | \(2.8 \times 10^{-39}\) |
| 硫化铁 | \(\text{FeS}\) | \(6.0 \times 10^{-18}\) |
| 氯化亚汞 | \(\text{Hg}_2\text{Cl}_2\) | \(1.3 \times 10^{-18}\) |
| 溴化亚汞 | \(\text{Hg}_2\text{Br}_2\) | \(5.0 \times 10^{-23}\) |
| 碘化亚汞 | \(\text{Hg}_2\text{I}_2\) | \(4.5 \times 10^{-29}\) |
| 硫化汞 | \(\text{HgS}\) | \(1.6 \times 10^{-52}\) |
| 碳酸锂 | \(\text{Li}_2\text{CO}_3\) | \(8.1 \times 10^{-4}\) |
| 碳酸镁 | \(\text{MgCO}_3\) | \(3.5 \times 10^{-8}\) |
| 氢氧化镁 | \(\text{Mg(OH)}_2\) | \(5.6 \times 10^{-12}\) |
| 氢氧化锰 | \(\text{Mn(OH)}_2\) | \(1.6 \times 10^{-13}\) |
| 硫化锰 | \(\text{MnS}\) | \(2.5 \times 10^{-13}\) |
| 碳酸铅 | \(\text{PbCO}_3\) | \(7.4 \times 10^{-14}\) |
| 铬酸铅 | \(\text{PbCrO}_4\) | \(2.8 \times 10^{-13}\) |
| 碘化铅 | \(\text{PbI}_2\) | \(7.1 \times 10^{-9}\) |
| 硫酸铅 | \(\text{PbSO}_4\) | \(1.6 \times 10^{-8}\) |
| 硫化铅 | \(\text{PbS}\) | \(9.0 \times 10^{-29}\) |
| 氢氧化锌 | \(\text{Zn(OH)}_2\) | \(3.0 \times 10^{-17}\) |
| 硫化锌 | \(\text{ZnS}\) | \(2.0 \times 10^{-25}\) |
常见难溶电解质的浓度积常数表
本文来自互联网用户投稿,该文观点仅代表作者本人,不代表本站立场。本站仅提供信息存储空间服务,不拥有所有权,不承担相关法律责任。如若转载,请注明出处:http://www.hqwc.cn/news/882672.html
如若内容造成侵权/违法违规/事实不符,请联系编程知识网进行投诉反馈email:809451989@qq.com,一经查实,立即删除!相关文章
换根 DP:进阶练习笔记
前言观前提醒:本文非新手向文章,不建议作为换根 DP 入门使用。
本文在洛谷专栏、博客园、CSDN同步发送。换根 DP 是树状 DP 的一种,思维难度较高,但是学会以后很套路也很轻松。
例题
P3047 [USACO12FEB] Nearby Cows G对于每个节点求出距离它不超过 \(k\) 的所有节点权值和…
clion 执行CMake 报错:Cannot read xxx\CMakeFiles\TargetDirectories.txt
在Windows下使用Mingw32编译,Clion 执行CMake时报错:D:\Develop\CLion-2021.1.3.win\bin\cmake\win\bin\cmake.exe -DCMAKE_BUILD_TYPE=Debug -G "CodeBlocks - MinGW Makefiles" D:\Work\C++Work\HelloWorld
-- The C compiler identification is GNU 8.1.0
-- Th…
官媒报纸下载器 | 快速下载电子版报纸
报纸下载器是一款可以快速下载电子版报纸的应用,无需安装双击即可使用。
它支持人民日报、农民日报、经济日报、中国证券报、工人日报、科技日报等多家官方报纸的在线下载。
可选择报纸并设置日期,点击下载按钮后,报纸将以PDF格式下载到本地。
下载进度和版面信息会在底部状…
RockyLinux操作系统
RockyLinux操作系统
1 系统介绍
2020 年 12 月 8 日,Red Hat 宣布他们将停止开发 CentOS,CentOS 一直是 Red Hat Enterprise Linux 的生产就绪下游版本,转而支持该作系统的较新的上游开发变体,称为“CentOS Stream”。作为回应,CentOS 的最初创办人 Gregory Kurtzer 在 Ce…
域名解析—互联网世界的导航系统
在互联网的世界里,每个网站都像一座“城市”,而用户要找到这些“城市”,必须依赖一套精准的导航系统——这就是域名解析。无论是浏览网页、发送邮件,还是使用移动应用,域名解析都在背后默默支撑着用户的每一次访问。本文将深入浅出地解析域名解析的原理、流程及其在互联网…
算法备案办理经验分享
算法备案实际办理经验,包含流程、材料和注意事项作为一名算法备案代办服务人员,之前又有一批客户通过了算法备案。趁着最近闲下来,今天就跟大伙分享下我做算法备案的经验,如有任何疑问,欢迎大家直接提问。一、算法备案流程
1.注册与主体信息提交
首先得登录互联网信息服务…
基于Ollama+DeepSeek+AnythingLLM轻松投喂打造本地大模型知识库.250212
第一步,下载开源的[AI]应用程序AnythingLLM
去官网Download AnythingLLM for Desktop下载并安装即可。
第二步,下载Ollama并获取DeepSeek LLM)
为了使用DeepSeek我们需要先下载Ollama并获取DeepSeek。
1、打开 Ollama 的官网http://ollama.com,在官网找到 “Download”,点击…
智慧消防网页低延迟播放RTSP视频流,低至300毫秒,25路不卡顿
在城市化进程加速的今天,消防安全已成为社会治理的核心课题。智慧消防通过物联网、人工智能、大数据等技术,构建实时感知、智能预警、快速响应的防控体系,但其核心环节——视频监控的实时性与可靠性,却长期面临技术瓶颈。传统消防监控系统依赖服务器转码方案,导致延迟高、…